
Actual Size:
3.5 MM x 18.3 MM
90-Day Sensor
Provides freedom from frequent self-insertions
4 Sensor changes/year
vs. 26-52 for other CGM Systems
Minimized maintenance
and management of supplies
Reduced number of
day-1 warm-up periods
The 90-day fluorescent sensor is inserted just under the skin of your patient's upper arm and continuously measures glucose for up to 3 months. We also provide you training on the simple, in-office insertion and removal procedures.

Actual Size:
3.5 MM x 18.3 MM
90-Day Sensor
Provides freedom from frequent self-insertions
4 Sensor changes/year
vs. 26-52 for other CGM Systems
Minimized maintenance
and management of supplies
Reduced number of
day-1 warm-up periods
Unlike traditional blood glucose sensors, the Eversense CGM Sensor is inserted subcutaneously in the upper arm by a trained health care provider. No part of the sensor shows through the skin surface.
Your nurse practicioner or physician’s assistant can also be trained to insert the sensor, making this advanced technology available to more patients.
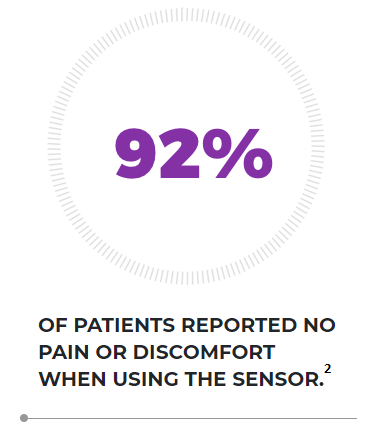
The Eversense sensor is made of a biocompatible material and it utilizes a patented fluorescent, glucose-indicating polymer technology to measure glucose in the interstitial fluid. The measurement is then relayed to the smart transmitter. The measurement and display of glucose values is done automatically, without the need for user intervention.
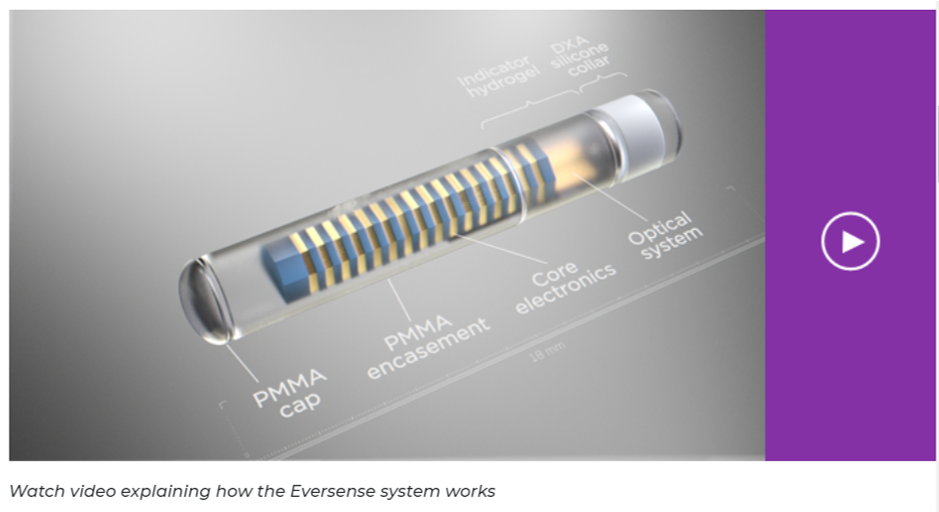

Advanced fluorescence technology
Sensor lasts up to 90 days - eliminates frequent self-insertions
Only CGM providing on-body vibe alerts when glucose is low or high
Very accurate over a 90-day period with MARD of 8.5%3
No fingersticks for treatment decisions