Up to 90-Days Sensor Wear
Transmitter provides
on-body vibe alerts
Fully implantable sensor
4 sensor changes a year vs 26-52 for other CGM systems
Exceptional accuracy – especially in the 40-60 mg/dL range2
Eversense is the only CGM system with a 90-day subcutaneous sensor – giving patients the longest wear time ever, along with unmatched convenience and accuracy.1,2,3
Now you can provide your patients with innovative CGM technology that can simplify their lives and provide better clinical outcomes. Eversense is the only CGM system with a 90‑day, subcutaneous sensor giving patients the longest wear time ever, along with unmatched convenience and accuracy.1,2
Up to 90-Days Sensor Wear

Transmitter provides
on-body vibe alerts

Fully implantable sensor

4 sensor changes a year vs 26-52 for other CGM systems
Exceptional accuracy – especially in the 40-60 mg/dL range2
Patients with diabetes receive optimal clinical benefit from continuous glucose monitoring when the sensor is worn more than 80% of the time.4
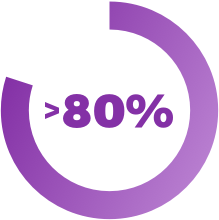
There are many limitations that result in patients struggling to continue using traditional CGM technology.6
Over 25% of users discontinue use within a year.7
Within a 10-minute window, the Eversense CGM System uses threshold and predictive alerts to detect:2
95% of hypoglycemic events (70 mg/dL)
99% of hyperglycemic events (180 mg/dL)

In addition to visual and auditory alerts, Eversense’s unique on-body vibe alerts add an extra measure of confidence, especially when patients are sleeping, or when their mobile device is not close by.
Multiple clinical trials have demonstrated that Eversense CGM use can reduce A1c levels.1

Up to 90-day sensor life
4 sensor changes per year
Only 4 ‘Day 1' warm-up periods
Sensor insertion by HCP
Transmitter is removable and provides unique on-body vibe alerts
Sensor stays put no matter what
Reduced irritation with gentle, silicone-based adhesive7
Short sensor life of 7-14 days
26-52 sensor changes per year
26 - 52 ‘Day 1’ warm-up periods
Need to self-insert the sensor
Transmitter must remain adhered to skin for sensor duration
Percutaneous sensor can be dislodged during everyday activities
Acrylate-based adhesives can cause skin irritation
Ready to prescribe Eversense CGM? Just fill out a simple form with a few details and we will contact you to answer questions and get you started.
