
Actual Size:
3.5 MM x 18.3 MM
No More
Inconvenient or Painful Self-Insertions Every 14 Days
No More
wasted sensor when transmitter is removed
No More
Worrying About Losing a Sensor
No More
Overtaping required for adhesive to last for days
The Eversense sensor sits just under the skin – providing 90 days of highly accurate CGM.1

Actual Size:
3.5 MM x 18.3 MM
No More
Inconvenient or Painful Self-Insertions Every 14 Days
No More
wasted sensor when transmitter is removed
No More
Worrying About Losing a Sensor
No More
Overtaping required for adhesive to last for days
The tiny fluorescent sensor is placed just under the skin of your upper arm by a trained Health Care Provider. There’s a small incision which is then closed with Steri-StripsTM. Most people heal in a few days. And then you’re good to go for up to 3 months.
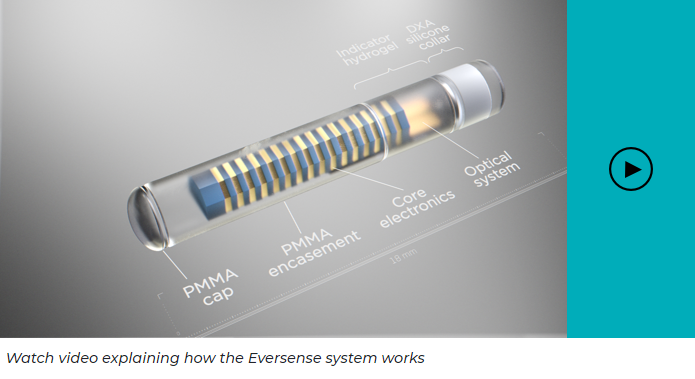
Advanced fluorescence technology
Sensor lasts up to 90 days – eliminates frequent self-insertions
Only CGM providing on-body vibe alerts when glucose is low or high
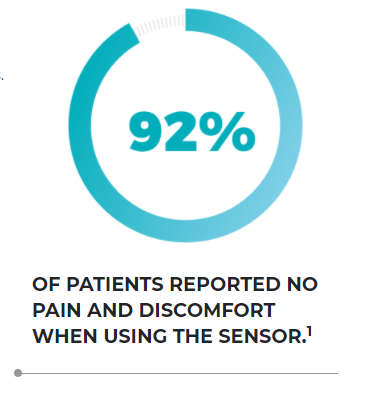
Very accurate over a 90-day period with MARD of 8.5%1
The Eversense sensor is encased in material which is compatible with the human body, or bio-compatible. It uses a patented fluorescent glucose-indicating polymer technology to measure glucose in the interstitial fluid (a thin layer of fluid that surrounds the body’s cells). The measurement is then relayed to the smart transmitter. Both the measurement and calculation of glucose values are done automatically every 5 minutes.
Ready to be Unstoppable with Eversense?
To get started, fill out a simple form with a few details, and we'll contact you to begin your order.
Madison, Eversense CGM user
Madison, Eversense CGM user