Experience A Better Way To Manage Your Diabetes
Eversense Has Been A Real Game Changer For Mateo
Watch how Eversense helps Mateo get all the confidence, flexibility and peace of mind he needs to provide the attention his students deserve.
Break Free From
- The weekly or bi-weekly hassle of sensor changes and site maintenance
- Accidental sensor dislodging
- Wasting sensor when transmitter is removed and replaced
- Ongoing CGM supplies to carry and order
- Concerns about an adhesive having to last up to 14 days
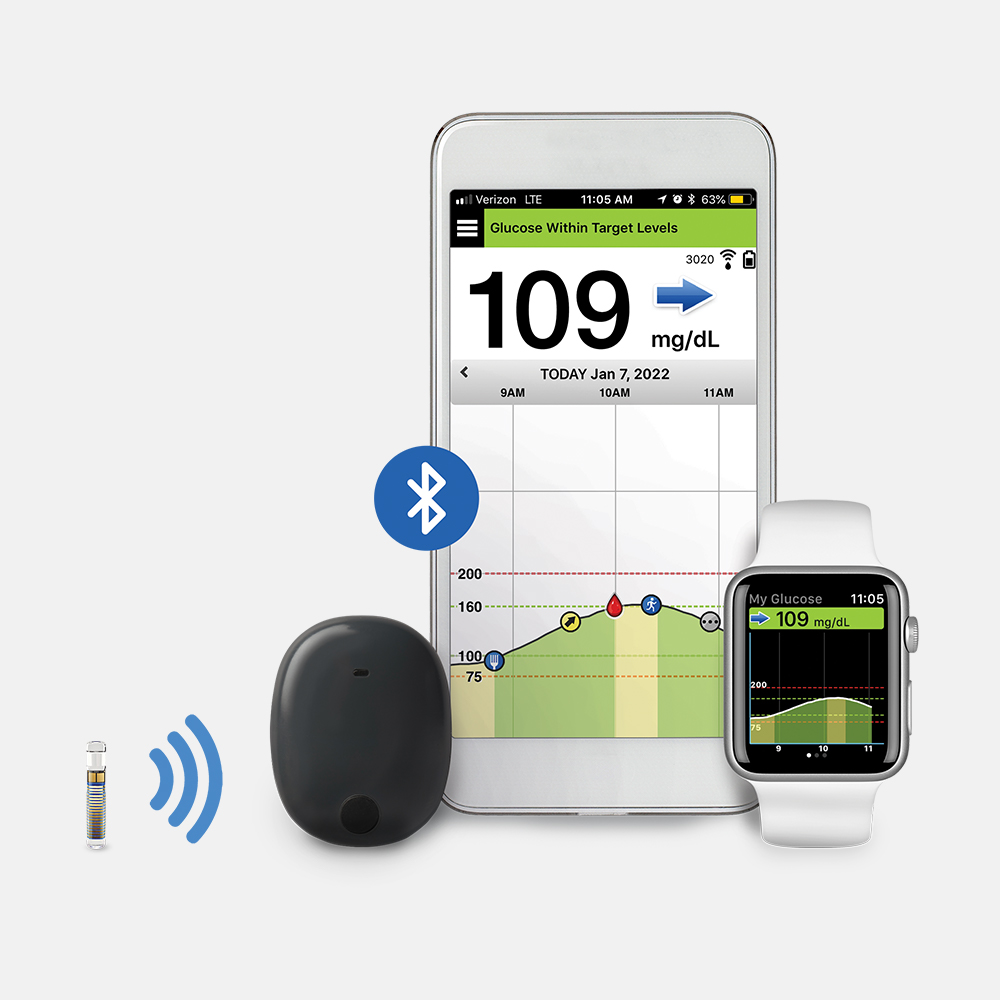
Eversense And Other CGMs
Other CGMs

Short sensor life of 7-14 days

26-52 sensor changes per year

26-52 ‘Day 1’ warm-up periods

Need to self-insert the sensor

Transmitter must remain adhered to skin for sensor duration

Percutaneous sensor can be dislodged during everyday activities

Acrylate-based adhesives can cause skin
irritation
Eversense

6-month sensor life

2 sensor changes per year

Only 2 ‘Day1’ warm up periods

Sensor insertion by an Eversense Inserter

Transmitter is removable** and provides unique on-body vibe alerts

Sensor stays put no matter what

Reduced irritation with gentle, silicone-based adhesive1
Clinically Proven
Accurate Through 6 Months2
Accurate Through 6 Months2
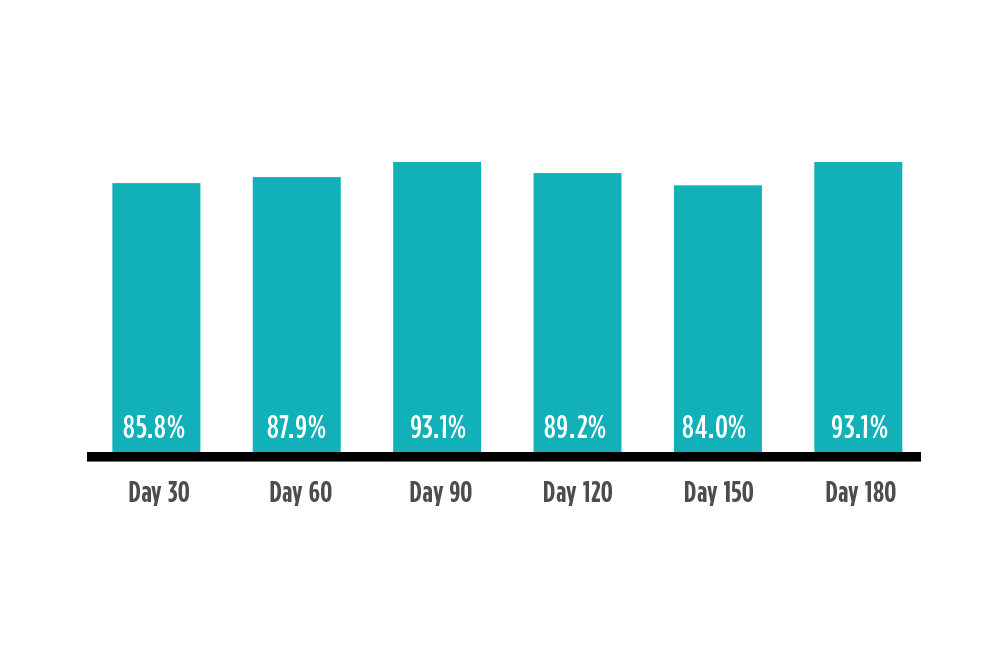
Multi-site prospective pivotal clinical trial of 180 type 1 and type 2 diabetes participants.
Accurate, Stable Performance up to 6 Months2
Percent of readings within 15 mg/dL or 15% of reference value
SENSOR DAYS
Proven high users acceptability
85%
Reported improved confidence over control of their diabetes3
86%
Reported less day-to-day burden3
80%
were more motivated to keep up with diabetes management3
84%
of users would choose to be inserted again3


*Up to 6 months
**There is no glucose data generated when the transmitter is removed
***The opinions expressed belong solely to the individual. This information provides general information only. It is not intended to be used as medical advice, diagnosis or treatment and should not replace the advice of a Health Care Provider
MARD is the mean absolute relative difference between sensor readings and matched reference values; the lower the MARD, the better the accuracy of the sensors being evaluated
1. Sanchez P, Ghosh-Dastidar S, Tweden K, Kaufman F. Real-World Data from the First US Commercial Users of an Implantable Continuous Glucose Sensor. Diabetes Technol Ther. Published online August 12, 2019, DOI:10.1089/dia.2019.0234.
2. Garg, S. K. et al. (2021). Evaluation of Accuracy and Safety of the Next-Generation Up to 180-Day Long-Term Implantable Eversense Continous Glucose Monitoring System: The PROMISE Study. Diabetes Technology & Therapeutics, 24(2), 1–9. DOI: 10.1089/dia.2021.0182.
3. Barnard et al. Acceptability of Implantable Continuous Glucose Monitoring Sensor. Journal of Diabetes Science and Technology 2018, Vol. 12(3) 634–638.


