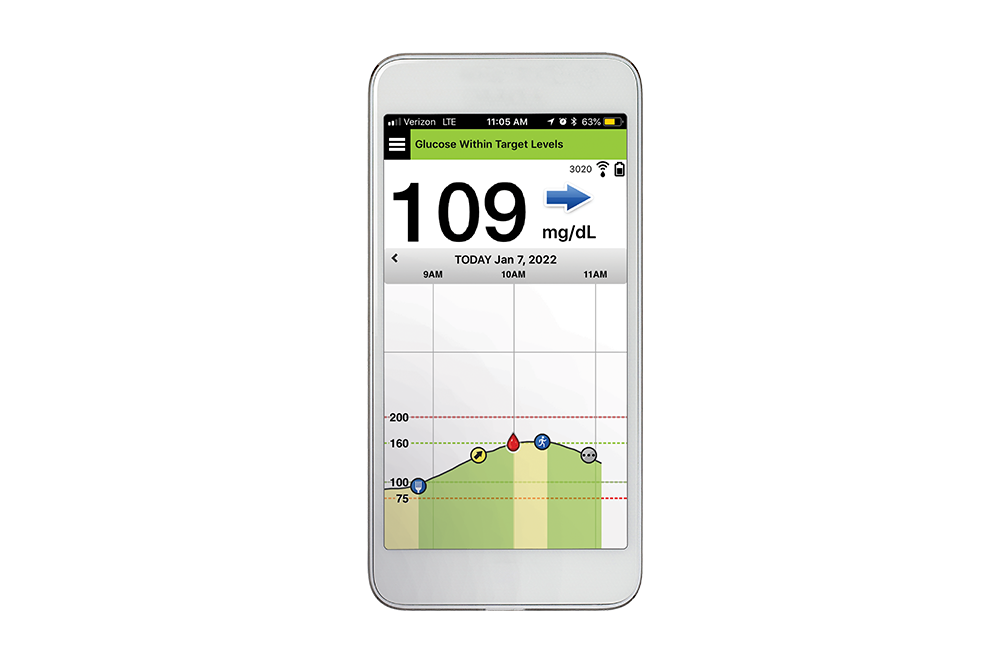
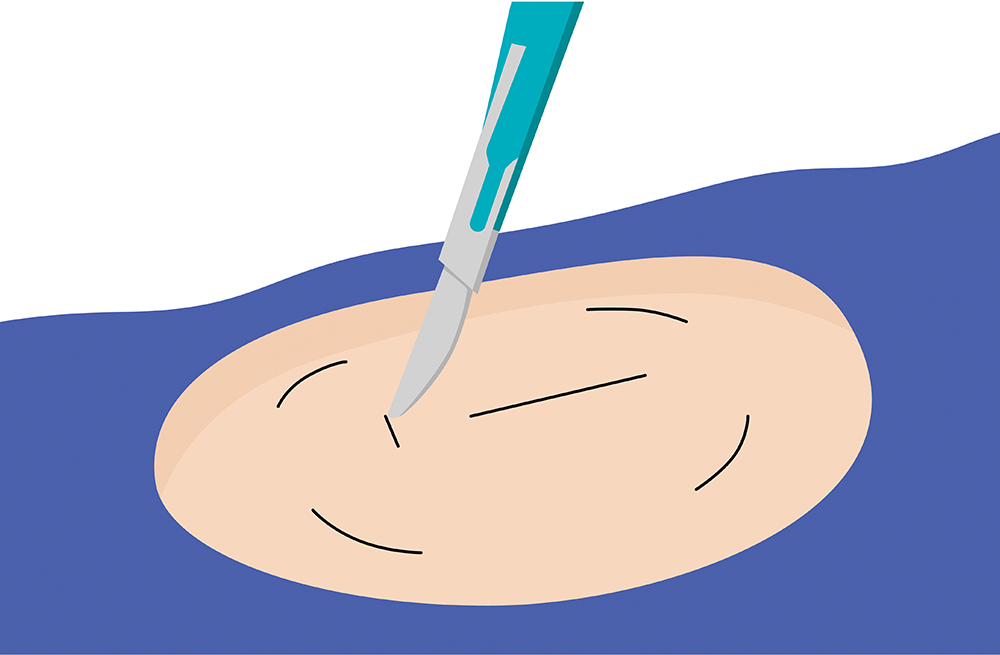
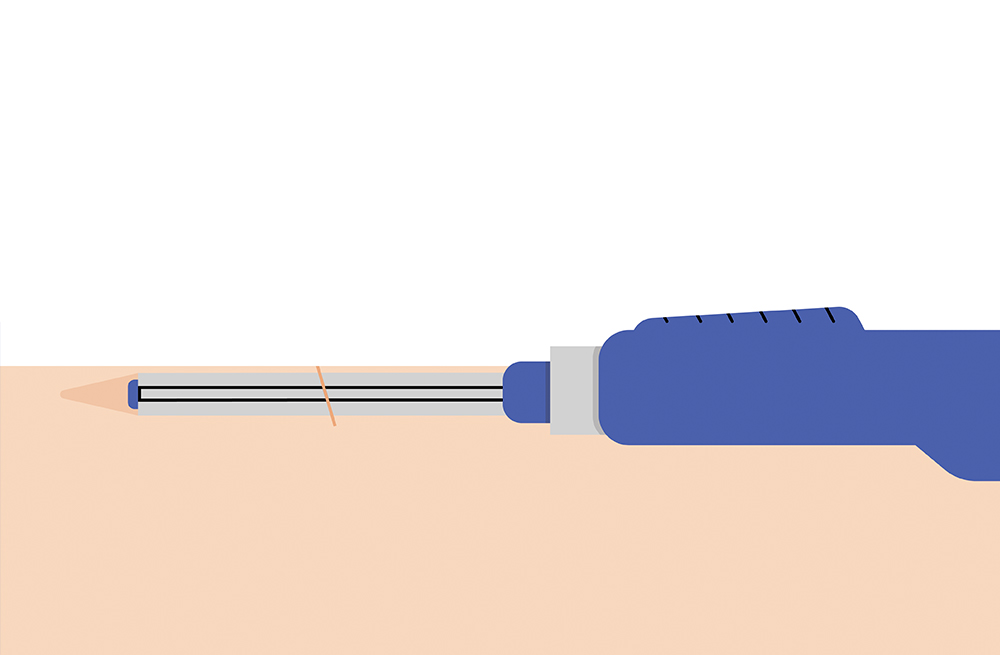
The sensor is inserted by an Eversense Inserter in the upper arm and continuously measures glucose for up to 6 months.
Changing The Way Diabetes Is Managed
Eversense E3 is the only CGM system with a 6 month implantable sensor — giving patients the longest wear time ever, along with exceptional accuracy up to 6 months. Increase your patients’ satisfaction and utilization and help them break free from the burden of frequent, inconvenient and sometimes painful self-insertions.
The 6-Month Eversense CGM System
The Only Implantable Sensor For Long-Term Wear
While all CGM systems use transcutaneous sensors that are inserted into the skin and last 7-14 days, the Eversense sensor is inserted completely under the skin. The Eversense CGM system lasts up to 6 months — eliminating the hassle and discomfort of frequent sensor insertions. Simply put, it makes managing your diabetes so much easier.
Solving The Unmet Needs Of Cgm Technology
Patients with diabetes receive clinical benefit from continuous glucose monitoring when the sensor is worn more than 70% of the time.1 Limitations of CGM technology have led some to discontinue use after just the first year.2
Current CGM users cited key improvements they desire.3
- "Longer sensor wear"
- "Better accuracy"
- "Better sensor adhesive"
Clinically Proven Accurate Through 6 Months4
Multi-site prospective pivotal clinical trial of 181 type 1 and type 2 diabetes participants.

Only 2 sensors per year with
Eversense E3 vs 26-52 sensor changes with traditional CGMS
8.5% Overall MARD4*
Proven high user acceptability
85%
Reported improved confidence over control of their diabetes5
86%
Reported less day-to-day burden5
80%
were more motivated to keep up
with diabetes management5
84%
of users would choose to be inserted again5


Contact Us
To learn more about Eversense CGM and how to get started:
Give us a call at 1-844-736-7348
(844-SENSE4U)
Email us at
eversense.us@ascensia.com
*Eversense E3 CGM Systems include the Eversense E3 Sensor with sacrificial boronic acid (SBA) design modification. In the PROMISE study, MARD of 8.5% was observed in the E3 Sensor with SBA, and MARD of 9.1% was observed in primary sensor without SBA design modification.
†On a compatible android or iOS device. For a full list of compatible devices, please click here.
**There is no glucose data generated when the transmitter is off
***The opinions expressed belong solely to the individual. This information provides general information only. It is not intended to be used as medical advice, diagnosis or treatment and should not replace the advice of a Health Care Provider.
1. Battelino T, Danne T, Bergenstal R, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From The International Consensus on Time in Range. Diabetes Care 2019; 42: 1595-1597. doi: 10.2337/dci19-0028.
2. Yu, S., & Varughese B, et al. (2018). Healthcare resource waste associated with Patient Nonadherence and Early discontinuation of Traditional continuous glucose monitoring in Real-world Settings: A MULTICOUNTRY ANALYSIS. Diabetes Technology & Therapeutics, 20(6), 420-427. doi: 10.1089/dia.2017.0435.
3. dQ&A Q1 2021 Panel survey.
4. Garg S. et al.. Evaluation of Accuracy and Safety of the Next-Generation Up to 180-Day Long-Term Implantable Eversense Continuous Glucose Monitoring System: The PROMISE Study. Diabetes Technology & Therapeutics 2021; 24(2): 1-9. DOI: 10.1089/dia.2021.0182.
5. Barnard et al. Acceptability of Implantable Continuous Glucose Monitoring Sensor. Journal of Diabetes Science and Technology 2018, Vol. 12(3) 634–638